Applications
Technology for particle size analysis
There are a wide range of different technologies available for particle size analysis, in particular for particles suspended in fluid. Here we discuss methods used to size particles in the range of 10 nm-10 μm in diameter, comparing Spectradyne’s nCS1TM, which uses microfluidic resistive pulse sensing (MRPS), to other commercial technologies. We also discuss fluorescent phenotyping combined with MRPS, as deployed in Spectradyne’s ARCTM. For comparison, virus particles are typically in the 50 nm range and up; extracellular vesicles, which includes exosomes, range from a few tens of nm up to several microns in diameter; red blood cells are 6-8 μm in diameter and white blood cells such as neutrophils are in the 10-12 μm diameter range; a human hair is about 100 μm in diameter. Particles larger than this are most easily studied using optical microscopy, and are not discussed here.
The methods available include:
Below we briefly discuss each one of these technologies and list the merits and shortcomings of each. One important thing to remember when discussing particle size analysis is that each technique reveals a certain aspect of the sample under study, and using more than one method, especially ones that use distinct technologies (also called orthogonal methods), is significantly more likely to yield important information about your samples that will not be apparent when using just one method.
If you’re interested in learning more, we have a wide range of literature available in our library.
“The nCS1 is fast, accurate, and easy to use for routine EV quantification. We looked at other nanoparticle measurement solutions and the nCS1 is the only instrument that could measure the size of every nanoparticle of interest in as little as 3 uL of volume. Moreover, their customer service is superb and have went out of their way to ensure our nCS1 is always functioning well.”
Joseph Sedlak, Co-Founder at Mercy BioAnalytics
Electrical sensing
Electrical methods include resistive pulse sensing or RPS, and its microfluidic cousin, microfluidic resistive pulse sensing or MRPS. These both use electrical signaling to measure particles suspended in fluid one at a time, as the particles pass through a pore between two fluid-filled volumes. Spectradyne’s nCS1TM technology uses microfluidic resistive pulse sensing implemented using a disposable microfluidic cartridge.
These two techniques are described in more detail below.
Spectradyne’s nCS1TM and ARCTM
Microfluidic resistive pulse sensing (MRPS)
Microfluidic resistive pulse sensing (MRPS) is the microfluidic cousin of resistive pulse sensing or RPS. The main difference is that the analysis chamber in RPS is replaced by a disposable microfluidic cartridge, where different cartridge models are designed to have different size apertures, allowing a wider range of particle sizes to be analyzed. This means that the cartridge chosen for the particle size analysis can be optimized for the particle sizes expected in the sample.
Spectradyne’s nCS1TM and ARCTM employ microfluidic resistive pulse sensing using disposable microfluidic cartridges. By using state-of-the art microfluidic fabrication technology, we have shrunk the critical particle sensing constriction used in traditional RPS by a factor of 100 or so, from a few tens of microns in diameter to a few hundred nanometers. This allows us to detect much smaller particles using MRPS than is possible using RPS. A schematic of a microfluidic cartridge is shown below. The microfluidic cartridges include a 3 μL reservoir for the analyte, on-board filters, ports for voltage-biasing the analyte, a sense electrode, a fluid resistor and the critical MRPS aperture or nanoconstriction, through which particles flow one at a time. The analyte, which could be blood serum, diluted or full-strength phosphate-buffered saline, or any conducting fluid, is electrically biased by the voltage-bias electrodes, causing an electrical current to flow through the analyte, the fluidic resistor and the aperture. When a particle suspended in analyte flows through the aperture, it changes the electrical resistance of the constriction by occluding part of the current, by an amount proportional to the ratio of the nanoparticle volume to that of the aperture, just as in RPS. Individual particles thus give changes in the sense voltage proportional to their volume, allowing them to be counted and sized. The much smaller apertures available in
Spectradyne’s microfluidic cartridges allow much smaller particles to be detected.
The disposable MRPS cartridge includes ports for voltage bias Va and Vb, a microfluidic nanoconstriction or aperture Ra and a fluidic resistor Rb, The voltage bias causes a current I to flow, setting the voltage Vout of the sense electrode. Particles flowing through the aperture change the aperture resistance in time (top right), thereby causing measurable changes in the sense voltage Vout. These changes are proportional to particle volume, so each particle’s volume is measured individually.
You can read more about microfluidic resistive pulse sensing elsewhere on our website:
- An overview of our technology
- Compare with the other particle size analyzer technologies
- Spectradyne’s MRPS allows measurements over a wide range of concentrations
- Specifications for Spectradyne’s nCS1TM
- An article published in American Laboratory is available here
- A technical article that appeared in Nature Nanotechnology is available here

A microfluidic cartridge at the heart of Spectradyne’s MRPS technology.
Spectradyne’s nCS1TM, which performs measurements using a microfluidic cartridges.
Spectradyne’s ARCTM
Fluorescence phenotyping combined with MRPS
Almost all biological nanoparticle samples contain heterogeneous populations of many different particle types. Spectradyne’s ARC can measure the size and concentration of the entire distribution using MRPS, and simultaneously measure and display only those particles in the sample that are positive for fluorescence in up to three different fluorescent channels.
The diagram above illustrates how the combination of MRPS and fluorescence phenotyping is implemented in Spectradyne’s ARC instrument. Nanoparticles flowing through the central nanoconstriction in a microfluidic cartridge are measured by MRPS, using electrical signaling; at the same time, a laser illuminates the nanoparticle, and any fluorescent signal is collected and analyzed by instrument optics; the intensity and fluorescent wavelength are recorded and combined with the measurement signal from the MRPS analysis, providing combined data that allows e.g. identification and sizing of a nanoparticle.

Spectradyne’s ARCTM, combining fluorescence phenotyping with MRPS.
The Spectradyne advantage
The Spectradyne nCS1 is the only benchtop technology that provides high-resolution size distributions and accurate concentration measurements for particles in the 50 nm – 10 μm diameter size range. The instrument, using only electronic sensing with no optical elements, rapidly counts and sizes individual nanoparticles in a sample, achieving few-percent precision in both size and concentration. The nCS1 delivers unprecedented capabilities for analyzing nanoparticles of any type, yielding more accurate and representative results than any other method. It thus provides an orthogonal technique to optically-based microparticle analysis instruments.
Read our overview of the technology, check out our specifications, as well as our comparative head-to-head comparison with other technologies.
Watch a video presentation that gives an overview of Spectradyne’s technology.
Resistive pulse sensing (RPS)
Resistive pulse sensing (RPS) is the particle size analysis method used worldwide to quantify red and white blood cell counts in whole blood analysis, where it is also known as Coulter counting. The method relies on the electrical sensing of the size of particles. A sample volume is separated into two sides by an impermeable membrane in which a small opening has been made. Both sides of the volume are filled with a weakly electrically-conducting fluid (such as saline or other biologically-compatible solution); one side of the volume also has the particles to be analyzed. A small pressure difference is applied across the aperture, causing fluid, and the particles suspended in it, to pass through the aperture. An electrical voltage bias is simultaneously applied across the aperture, causing a small electrical current to pass through the aperture. When no particle is passing through the aperture, the electrical current has one value; when a particle passes through the aperture, it changes the volume through which the electrical current can flow, so the electrical resistance of the aperture increases. The electrical current is correspondingly reduced, by an amount that turns out to be strictly proportional to the volume of the particle.
RPS is thus a single particle method: Each particle that passes through the aperture momentarily changes the electrical current by an amount proportional to the particle volume, for a time inversely proportional to the speed of the particle, and thus the flow rate of the fluid. By counting particles and measuring the flow rate, the particle concentration can be very accurately determined; by binning the volumes of the particles as they pass through, a quantitative analysis of the number of particles as a function of particle volume can be calculated.
These data form the basic output for a particle size analysis performed by RPS: Particle concentration versus particle size. As each particle is sensed individually, there are no assumptions about how the signal that is detected is related to the number or size of each particle: The measurements are direct. RPS instruments are benchtop instruments, and sample preparation is minimal. The size range of particles that can be analyzed using RPS depends on the size of the aperture and the sensitivity of the electronics used to detect the particles; typically the dynamic range (in terms of particle diameter) is from about 1 μm up to 100 μm. In standard RPS, the two volumes containing the fluid, and the aperture that connects them, is a permanent part of the instrument, and is not normally disposable; this thus allows for some sample cross-contamination. However, in microfluidic-RPS (MRPS), which we discuss below, this central particle analysis part of the instrument is replaced by a disposable microfluidic cartridge, eliminating this potential source of error.
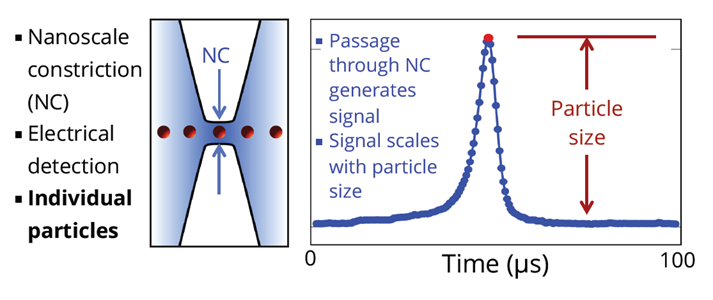
Particles in fluid pass through a constriction (NC) as shown on left side of the drawing above. A voltage is applied continuously across the two sides of the NC. As particles pass through the NC, the output signal changes in proportion to the volume of the particle. Particles are measured individually, with no dependence on particle material.
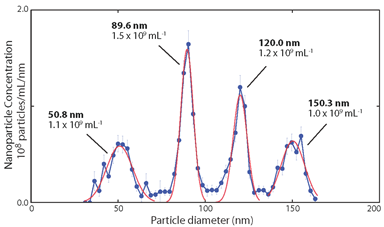
Typical data achievable using a RPS instrument for particle size analysis, with quantitative vertical and horizontal axes accumulated from single particle measurements, with good particle size resolution.
Light scattering methods
Light scattering methods include dynamic light scattering or DLS, which is a bulk measurement technique, and nanoparticle tracking analysis or NTA, which tracks individual particles as they move due to Brownian motion in the suspending fluid. These are each described below.
Dynamic light scattering (DLS)
Dynamic light scattering (DLS) is a technique that measures large populations of particles (it is not a single particle-by-particle technique). A laser is used to illuminate the fluid sample in which the particles are suspended, and the laser light scatters off the particles. Because the light from a laser is coherent, the scattered light from different particles interferes and creates a projected pattern of light and dark regions (called a speckle pattern). Photodetectors off to the side of the laser axis are used to catch and monitor the time dependence of this speckle pattern, where software generates from these data the scattered light auto-correlation function. By analyzing the time dependence of the auto-correlation function, in particular the time dependence of the decay in the correlation of light, the size of the particles can be inferred using the physics of Brownian motion.
The mathematics behind this analysis is complicated, and many assumptions are made to arrive at the final result, which works best if the sample is monodisperse, meaning there is only one size of particle in the sample; it works much less well if the sample is a mixture of different particle diameters. Part of the problem is that larger particles scatter much more light than small particles; the amount of scattered light scales as the sixth power of the diameter, D6, so a particle with twice the diameter scatters 64 times as much light, and thus dominates in the analysis of the sample. DLS also works best if the sample has a low particle concentration, so the laser only scatters on average once off a particle before reaching a detector.
DLS requires little sample preparation, and the instrument is a benchtop tool. However, interpreting what the measurement reveals about the sample is fraught with uncertainty, and can be very misleading. As often little is known a priori about the actual distribution of particle sizes in the sample, using DLS with no orthogonal method is highly risky.
Read here to see how DLS compares to Spectradyne’s MRPS technology.
This application note will tell you about false peaks in DLS measurements.
A laser speckle pattern.
Nanoparticle Tracking Analysis (NTA)
Nanoparticle tracking analysis (NTA) is very similar to dynamic light scattering or DLS; it is essentially its single-particle cousin. A special sample holder is filled with the fluid sample in which the particles are suspended, and a laser illuminates the sample at grazing incidence. The light scattered off the particles is collected by a microscope objective and projected onto a imaging electronics such as a CCD. Each particle forms an image on the CCD, and the motion of each particle is tracked as a function of time. The random Brownian motion is extracted from a time series of images, and from the physics of Brownian motion, the diameter of the particle (known as the hydrodynamic diameter) can be calculated, if the viscosity of the sample is known.
The method is simple and requires little sample preparation. This works well for particles in the size range of roughly 100 nm up to 1 μm (1000 nm); particles smaller than about 100 nm in diameter scatter significantly less light (the amount of scattered light scales as the diameter D to the sixth power), so smaller particles tend not to be detected, and particles larger than about 1 μm don’t move enough (their Brownian motion is too small) for their diameters to be extracted from the images. A single sample will yield a limited amount of information, as only a few hundred particles at most can be monitored at the same time; several sequential measurements are needed to get enough statistics to reliably report the particle concentration versus particle size. The sample holders are non-disposable and must be cleaned after each use to prevent sample cross-contamination.
The NTA method suffers from a curious problem known colloquially as the false peak problem. As the particle sensitivity to small particles falls as the sixth power of the diameter, the NTA will report a distribution that cuts off below a certain diameter, creating a false peak in the distribution. Using an orthogonal method can reveal this false peak, and is highly recommended for measurements where knowing the actual size distribution is important.
Read more about nanoparticle tracking analysis.
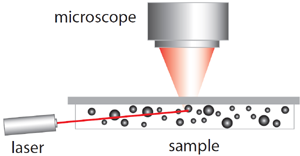
Diagram of the measurement setup for NTA.
Electron microscopy
Electron microscopy is the technique that is either implemented as transmission electron microscopy or TEM, and scanning electron microscopy or SEM. The two techniques image individual particles dried from solution, and allow precise measurements of individual particles. These are described below.
Electron microscopy (TEM and SEM)
Electron microscopy, specifically transmission electron and scanning electron microscopy (TEM and SEM) are the gold standard for particle size analysis. To prepare a sample for electron microscopy, the sample is dried from a few milliliters of the fluid containing the particles. The sample is in some cases then coated with a nm-thick layer of conductive carbon or gold, to prevent spurious charging while viewing the sample. The dry coated sample is then placed in the sample analysis chamber of the microscope; most commonly this is in vacuum, although more expensive modern microscopes allow sample examination in air. The sample is then illuminated with a high energy beam of electrons generated by a cathode; the electrons can range in energy from a few kilovolts to a few hundred kilovolts, and are focused by coaxial magnets to a focus point 0.1 to 1 nm in diameter. This focus point is scanned over the sample in an SEM, while in a TEM the beam passes through the sample and the scattering occurs during the passage through the interior, after which scattered electrons are imaged on a CCD. Either the scattered electrons, or in some cases the secondary electrons generated by the primary electrons in the beam, or both, are collected and projected into an imaging system. Very high resolution images of particles can be formed in this way, and analyzed with precision measurement tools calibrated on the microscope using calibration standards. In a TEM, it is possible with crystalline materials to image the atoms that make up the sample.
Particle diameters can be measured with great precision, and the geometric details of each particle are revealed in the images. By forming many images and using automated software, the size distribution of particles can be calculated and a concentration versus particle diameter can be calculated.
The sample preparation and sample imaging as well as image analysis are very time-consuming. TEMs and to a lesser extent SEMs are large, room-filling pieces of equipment that need special spaces with low vibration, low stray magnetic fields, and good temperature control. Using this method as a regular sample measurement technique is clearly prohibitive in terms of both cost and time. The data are typically used as an orthogonal verification of some of the other techniques discussed here.
A typical image of nanoparticles taken with a scanning electron microscope (SEM).
Mechanical methods
Mechanical methods include field fractionation techniques such as field-flow fractionation or FFF, in which particles are separated in fluid flow due to their differential mobilities, and disk centrifugation, where particles are rotated at high speeds in a disk and separate due to their differential motion in a centrifugal force field. These are each described in more detail below.
Field flow fractionation
Field fractionation techniques separate particles suspended in a fluid sample using what is called a “field,” where under the action of the field, different particles move at different rates. The field used for the separation depends on the particular instrument; it can be a gravitational field, a centrifugal field, a thermal gradient, a magnetic force, and so on.
Field flow fractionation (FFF) provides the best known method for field fractionation. Fluid containing the particles to be analyzed is flowed through a long channel whose sides are formed by semi-permeable membranes. A gentle perpendicular fluid flow is passed through these membranes, causing a slow drift in the particles moving along the channel, where larger particles move slightly faster in this cross-flow than smaller particles, due to their larger hydrodynamic radius and thus larger drag coefficient. At the output of the channel, there is as a result a gradient of particle sizes across the width of the channel, and this gradient is detected, typically using an optical imaging method, which measures the relative concentration of different diameters across the channel width. This is not a single particle-by-particle method, as it measures a bulk response. Quantitative concentration measurements are difficult, but proportional particle number versus hydrodynamic radius information can be extracted.
The method requires little sample preparation and the instrument is a bench-top instrument. Cross-contamination of samples can occur as the analysis volume is not disposable. It is not a single particle method, so suffers from the usual challenges, yielding more proportional than absolute particle size and concentration information. It works for a wide range of particle sizes, which can be separated with diameter differences as small as 10%. However it can be confounded by the parabolic Poiseuille flow pattern in the main channel, where particles near the boundary flow more slowly and can accumulate on the downstream side of the separating flow, while on the opposite, upstream, side the particles cross into the higher flow rate near the center so separate by a different amount from particles that start in the channel center.
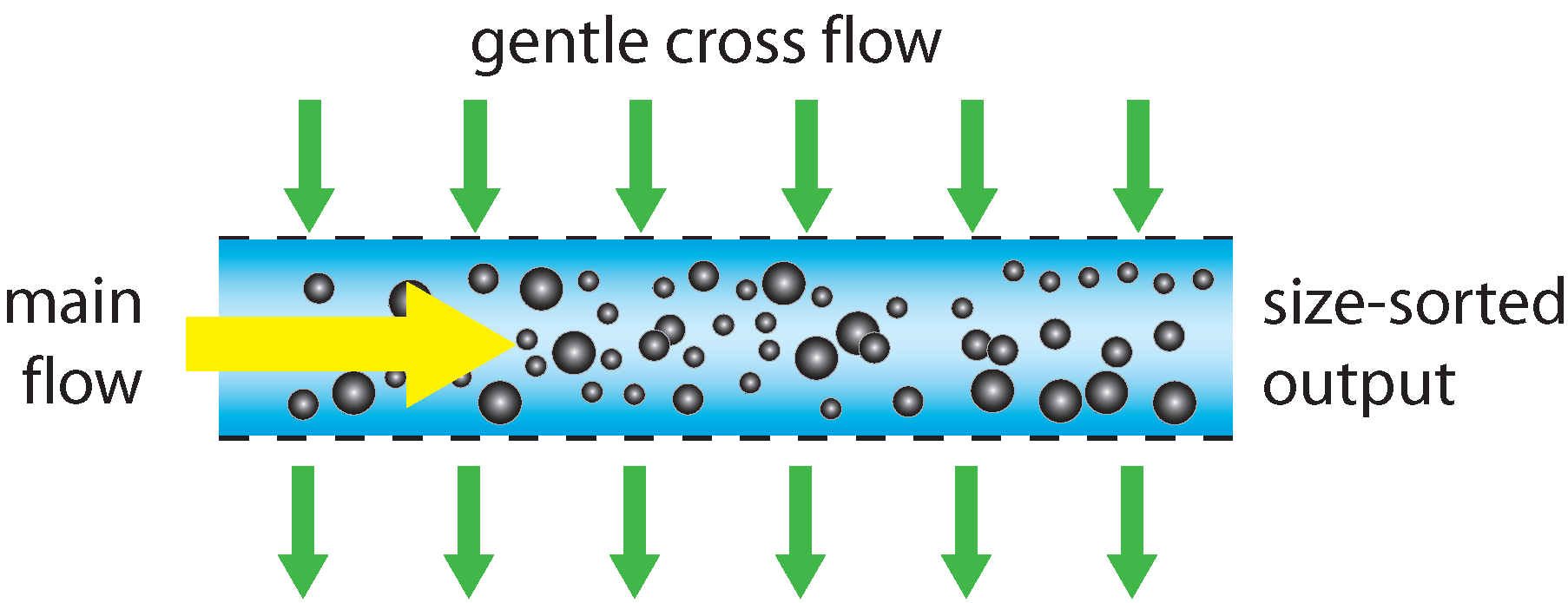
Schematic operation of a field-flow fractionation sorter.
Disk centrifugation
Disk centrifugation is another field fractionation technique. Field fractionation separates particles suspended in a fluid sample using what is called a “field,” where under the action of the field, different particles move at different rates. The field used for the separation depends on the particular instrument; it can be a gravitational field, a centrifugal field, a thermal gradient, a magnetic force, and so on.
In disk centrifugation, the sample is placed in the center of a rotating fluid-filled disk, which is then rotated about its axis at a high rotational velocity (typically 10,000-25,000 rpm), creating an effective gravitational field along the plane of the disk, many hundreds or thousands of times larger than the Earth’s gravitational field. If a particle in the sample has a higher density than the fluid in the disk, it will feel a net force in the direction of this field, due to the balance of the centrifugal force and the buoyant force of the fluid. It will then move at a velocity determined by the balance of the net force and the fluid frictional force, where the latter scales with the fluid viscosity and the particle’s hydrodynamic radius. A collection of particles with the same density but different diameters will thus separate in time because their terminal velocities (or mobilities) scale inversely with their diameters. When the particles diffuse to a certain diameter in the disk, they pass through a laser beam and obscure the light transmission to a detector on the other side of the disk. The degree of obscuration is proportional to the optical density of the particles (a combination of particle scattering strength and the number density of the particles), and the transmitted light intensity as a function of time allows the particle size distribution to be inferred.
A wide range of particle sizes can be analyzed using this technique, from maybe 10 nm to 10 μm. It is not a single particle technique, and only proportional concentration measurements can be made, due to the convolution of particle number density and optical density when monitoring the light transmission. Samples containing a range of different particle types are therefore difficult to analyzer. Quite high particle size resolution, maybe 5-10%, is possible. Sample preparation is minimal, but cross-contamination is possible because the disks are not disposable.
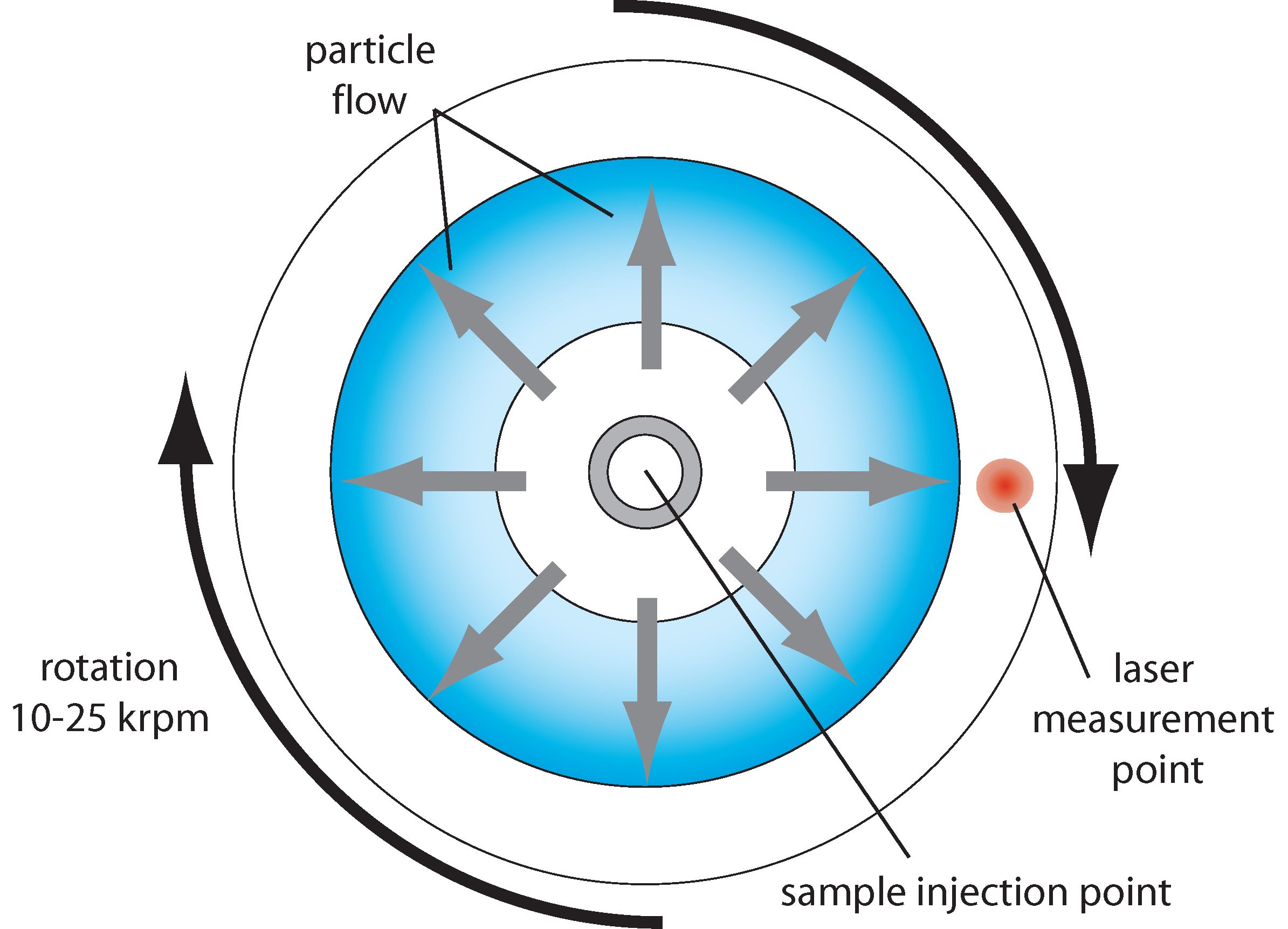
Schematic for a disk centrifugation particle analyzer.